Retinal Vascular Occlusions
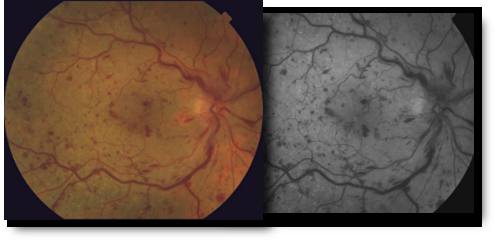
Branch & Central Vein Occlusions
The retina is the layer of tissue at the back of the inner eye that converts light images to nerve signals and sends them to the brain. Retinal arteries bring blood to the retina and retinal veins drain blood from the retina. When there is an occlusion, or blockage, of a retinal vein, there is back-up pressure in the capillaries, which leads to hemorrhaging and fluid leakage on the retina which causes vision loss.
Retinal vein occlusion is most often caused by hardening of the arteries (atherosclerosis) and the formation of a blood clot. Blockage of smaller veins (branch veins or BRVO) in the retina often occurs when retinal arteries that have been thickened or hardened by atherosclerosis cross over and place pressure on a retinal vein.
Branch vein occlusion are the most common type of vein occlusion, effecting males and females equally. Most occur after the age of 50, with individuals in there 60’s and 70’s having the highest occurrence. Specifically, aging, high blood pressure, diabetes, and smoking are all risk factors, so working with your internist or family practice physicians is important to control the underlying cause of the occlusio
