Dry AMD
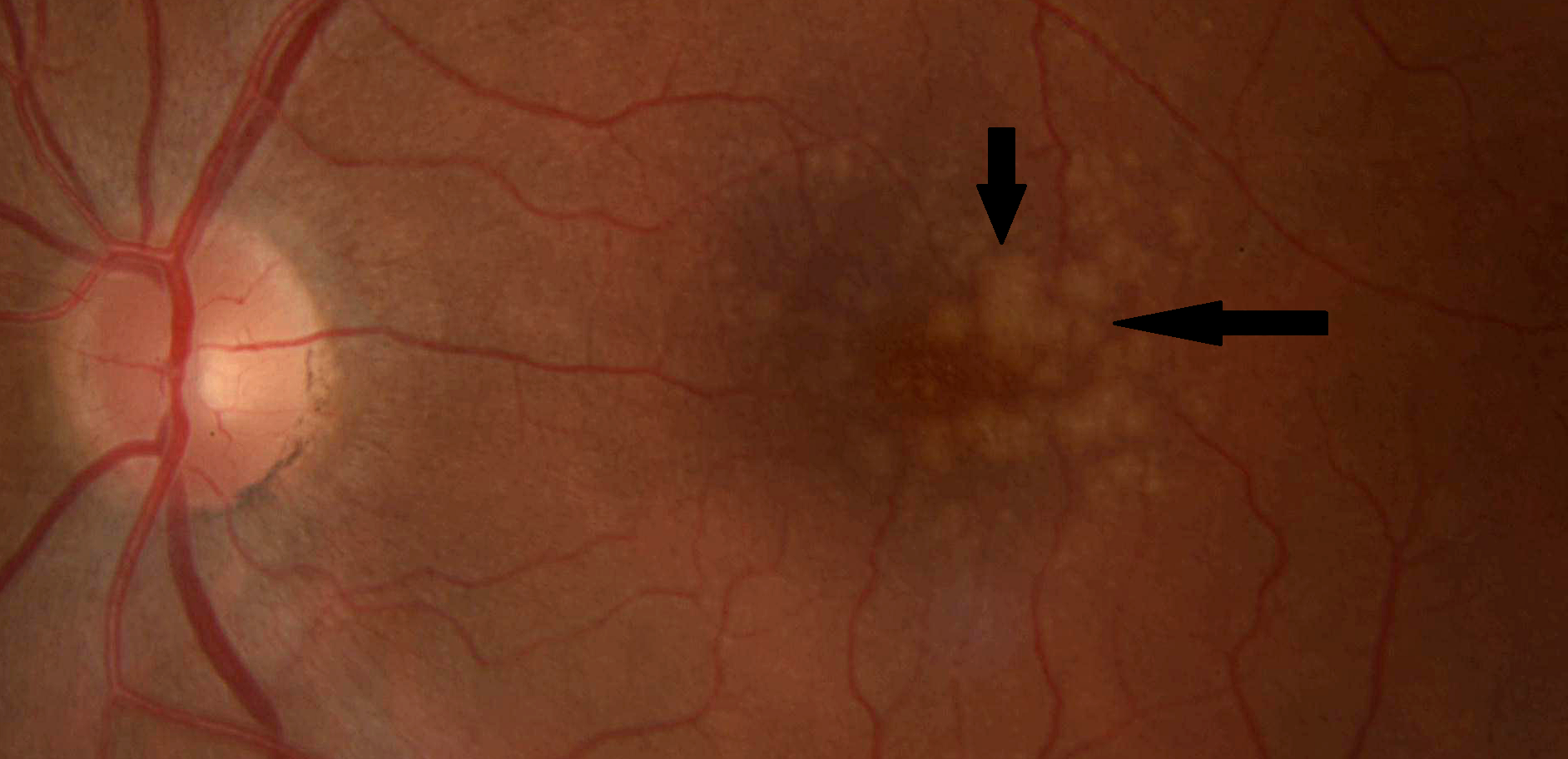
Drusen
Dry AMD affects about 85-90 percent of those with the disease. Its cause is unknown. Slowly, the light sensitive cells in the macula break down. The deterioration of the retina is associated with the formation of small yellow deposits (drusen) under the macula. With less of the macula working, you may start to lose central vision in the affected eye as the years go by. Dry AMD often occurs in just one eye at first. You may get the disease later in the other eye. Doctors have no way of knowing if or when both eyes may be affected.
If you have a family history you may be interested in a genetic test that can help determine the risk for developing the disease.
