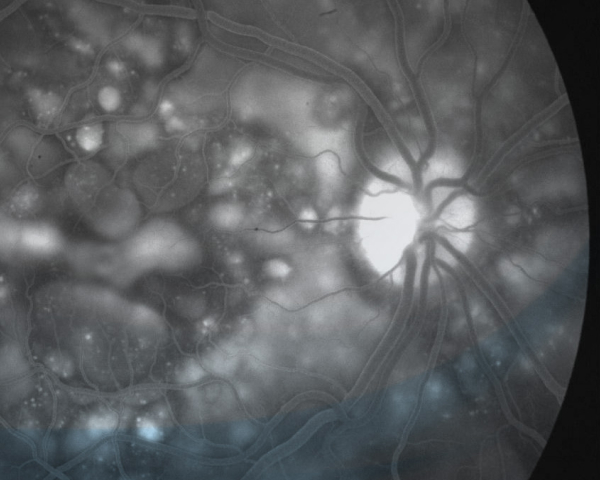
For Patients
We offer timely and sight-saving treatment for retina patients in California.
For Doctors
Over 2,000 unique referring physicians have entrusted their patients' care to us.
Welcome to California Retina Consultants
Our pioneering practice specializes in diseases and surgery of the retina, macula and vitreous. From 12 locations in Central California, we bring compassion and expertise to local patients while elevating global standards of care through research and clinical trials.
Specialized Retinal Care in Central California
In 1969, our practice was the first to provide vitreoretinal care to patients on the Central Coast of California. Today, we’re one of the largest and most highly-respected retina groups in the world.
We are committed to ensuring all patients have access to state-of-the-art, evidence-based diagnostics and treatment, and that those treatments are delivered in a personal, ethical, and caring manner.

13
top Physicians

12
COnvenient Locations

91
Patient Satisfaction (NPS) Score

1,032
Clinical Trials Enrollment
Memberships and Affiliations
“Everyone is so nice and caring.”

“Friendly staff and highly skilled and competent doctors!”

“The doctor is absolutely brilliant; she cares about people and knows her stuff.”

“Perpetually excellent, efficient service from skilled and friendly staff.”

Meet Our Retina Specialists
Through research, education, and patient care, the award-winning physicians of California Retina Consultants are working to reduce the number of individuals suffering from blindness and vision loss locally and around the world.
A Team of Experts:
Experience You Can Trust
Experience You Can Trust
California Retina Research Foundation
Since the founding of California Retina Research Foundation in 2001, we've been global leaders in the prevention of blindness through the advancement of research in vitreoretinal diseases. To date, our team has enrolled more than 1,000 patients in over 50 clinical trials.

Retina Consultants of America
California Retina Consultants is part of Retina Consultants of America, a network of leading retinal care and research practices in the United States. Together, we’re shaping the future of retinal and macula care and offering state-of-the-art treatment options.
Blog and News
Keep up with the latest news, updates, and announcements from California Retina Consultants.
![]() November 16, 2021
November 16, 2021Retinal Detachment Symptoms
![]() February 7, 2021
February 7, 2021AMD Awareness Month
![]() December 7, 2020
December 7, 2020Giving Back: The Gift Of Sight
![]() November 4, 2020
November 4, 2020November Is Diabetic Eye Disease Awareness Month
![]() August 29, 2019
August 29, 2019California Retina Consultants Opens Their Newest Location
![]() May 23, 2019
May 23, 2019How Smoking Affects Your Eyes