Age-Related Macular Degeneration
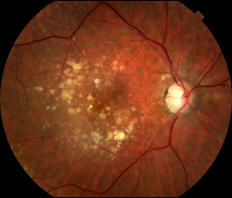
What is age-related macular degeneration?
Age-Related Macular Degeneration (ARMD or AMD) is the leading cause of vision loss in older American adults. AMD is also one of the most common reasons patients are seen at California Retina Consultants. Patients that have AMD will notice a loss of central vision, however retain good peripheral vision. This is due to the disease affecting the macular, which is the central part of the retina responsible for central vision, which also allows for the greatest amount of visual acuity or details to be seen.
Symptoms
Patients that have AMD can experience a wide variety of symptoms that usually begin gradually and painlessly. These symptoms include, but not limited to:
- Warping or distortion of strait lines or objects
- Increased difficulty in reading
- Blurred central vision
- Reduce central vision in one or both eyes
- Decreased intensity or brightness of colors
- Difficulty recognizing faces
Test Yourself with the Amsler Grid- Click HERE to learn more.
Who is at risk?
Age is a one of the major risk factors for AMD. AMD is most likely to occur after the age of 60, however it can develop at any age. Other risk factors include:
- Smoking- This has been shown to double the risk of AMD
- Family History- Genetics play a key factor. Patients with a family history of AMD or more likely to develop the disease.
- Race- AMD is most common in Caucasians
Although you can’t change your race or genetics, you can take steps to alter your lifestyle, which could possibly improve your chances of not developing AMD. This includes:
- Avoid smoking
- Exercise on regular basis
- Maintain good blood pressure and cholesterol levels
- Include leafy green vegetables and fish with omega 3 into your diet.
Is there more than one type of AMD?
Patients that are diagnosed with AMD are grouped into one of two categories, Dry AMD or Wet AMD. The most common type of AMD is the dry form, while 10-15% have the wet form of AMD. Most patients that have complete central vision loss have the wet form of the disease. AMD commonly starts as the dry form and in about 10-20% of patients, can progress to the wet form. Age-Related Macular Degeneration (AMD) forms in both eyes, but can progress at different rates in each eye. Because of this, it is possible to have both the wet form and dry form of the disease at the same time.
Click Below for more information or Dry or Wet AMD.
How is the AMD diagnosed?
AMD is a slow progressing and painless eye disease and the early signs of AMD usually start without any symptoms. That’s why it is important to have regular annual dilated eye exams by your doctor, especially if you are considered high-risk or have any or the previously mentioned symptoms. During you eye exam with our doctor, he/she will perform various clinical exams and tests to help properly diagnose the disease. The most two common test we perform is a Fluorescein Angiography (FA) and Optical Coherence Tomography (OCT).
A Fluorescein Angiography is the most common test used to determine if your form of AMD is the wet or dry form of the disease. During an FA, a small amount of a vegetable-based dye is injected through the arm or hand that lights up usually a special light. Next, our certified ophthalmic angiographer (retina photographer) with take a series of photographs of your retina, which will help your eye doctor determine if there is any leakage within your eye’s vascular system.
An Optical Coherence Tomography (OCT) is an innovative test that we can perform which is non-invasive, to look at various structures of the retina with an extremely high level of detail. An OCT allows our doctors to view cross sections of your retina, which helps them to properly detect, diagnosed and treat retinal diseases, such as AMD. Other retinal diseases such as macular edema, retinal vein occlussions and central serous chorioretinopathy can also be detected with the OCT.
What treatments are available for AMD?
California Retina Consultants has participated in dozens of FDA research studies, helping to pave-the-way for the treatment of various retinal diseases, including Age-Related Macular Degeneration. One popular treatment option is an injection designed to block a molecule called Vascular Endothelial Growth Factor or VEGF. VEGF causes the growth of leaky blood vessels in patients with the wet form of macular degeneration. Anti-VEGF injections are administered in the eye through a tiny needle. Patients might be apprehensive about having a “shot” placed within the eye, but most patients experience little to no discomfort with injections. Additional treatment options that aren’t quite as popular as Anti-VEGF injections include Photodynamic therapy, which uses a cold laser to treat select areas of the retina and laser surgery, which uses a hot laser to treat leaky blood vessels. Ask your doctor which treatment option would be best for you.
