Wet AMD
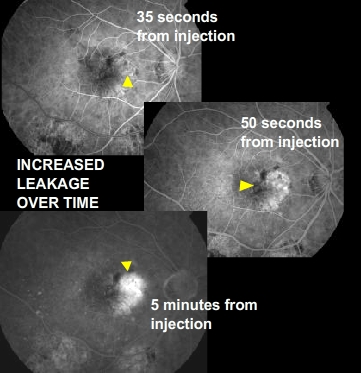
Although only 10 percent of all people with AMD have this type, it accounts for 90 percent of all severe vision loss from the disease. It occurs when new blood vessels behind the retina start to grow toward the macula. Because these new blood vessels tend to be very fragile, they will often leak blood and fluid under the macula. This causes rapid damage to the macula that can lead to the loss of central vision in a short period of time. This form of Macular Degeneration is the most threatening to your central vison. The degeneration of the RPE layer (atrophy) can cause a weakening of Bruchs membrane to a point that a blood vessel from the underlining choroidal layer can grow up and through the RPE. Like a weed growing through a crack in a sidewalk. This wet (exudative) form of AMD forms a sheet or mass of blood vessels refered to has a CNV membrane (Choroidal Neovascular). Choroidal, because that’s the point of orgin, Neovascular, “new” vessels, and membrane refers to the sheet or mass formation of these vessels. These new blood vessels are unlike regular blood vessels because they are weak and are prone to leak and break. This can cause an elevation into the surrounding tissue (RPE and retina) resulting in vision loss. Continued leakage will result in scar tissue formation, creating a permanent destruction of the tissue in
